I was recently reading Nobel Chemistry laureate, Roald Hoffman’s, really nice book on bonding in solids and thought that the explanations for certain concepts (like Peierls distortion and Bloch wavefunctions) are so much clearer than the way I was taught in physics, and also make it easier for me to teach. So I decided to make this video, introducing some useful concepts from this book as well as that I use in my work on a regular basis, such as antibonding orbitals, p-d models and Wannier models. You can click on the timestamps in my comment on the video to watch the most interesting parts for you: https://www.youtube.com/watch?v=x4ihhQrS6lA
If you are a condensed matter physicist or materials scientist and never took an inorganic chemistry class, you will likely find this useful (like I did). If terms terms like Wannier functions, antibonding and p-d model are a bit confusing, you'll probably also find this useful.
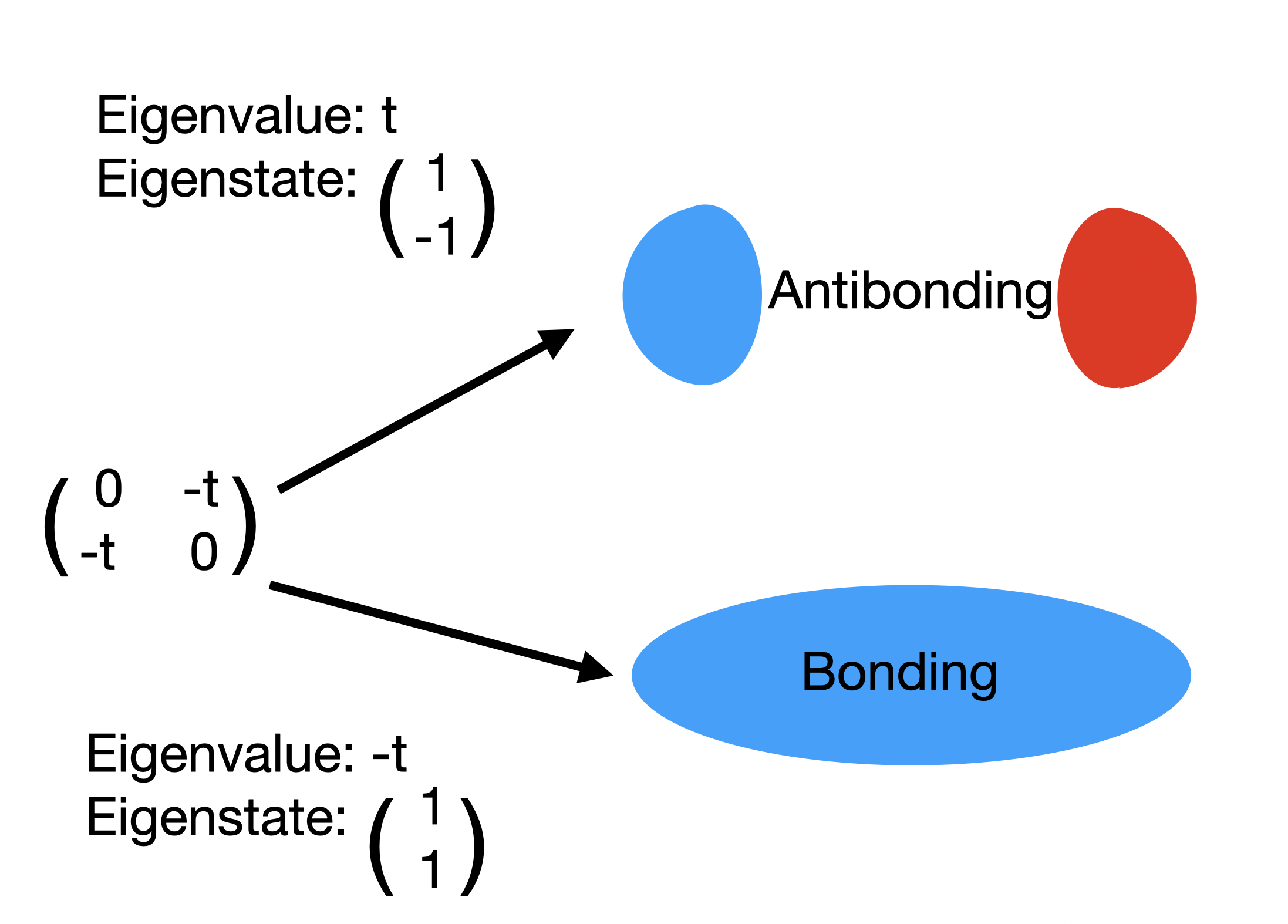
I discuss chemical bonding concepts as relevant for the Peierls distortion, bonding/antibonding (antibonding orbitals destabilize materials), Bloch wavefunctions, supercells/band folding, low energy vs p-d models, Wannier functions and the LCAO (linear combination of atomic orbitals) approximation.